Zainab Moustafa Tawfiq1, Ahmed Galal Yacout1, Sherif Mohammed El-Hadi1
1 Department of Anesthesia and Surgical Intensive Care, Faculty of Medicine, Alexandria University, Egypt
Corresponding Author:
Dr. Zainab Tawfiq
Specialist Anesthetist, Alexandria, Egypt
Email: zizitawfiq@gmail.com
ABSTRACT:
Background: Sugammadex is a synthetic modified γ-cyclodextrin derivative designed to bind selectively to the aminosteroidal neuromuscular blocking agents (NMBAs), such as rocuronium and vecoronium. Encapsulation of the rocuronium molecule by sugammadex results in a rapid migration of rocuronium away from the synaptic clefts back into the circulation. This promotes the liberation of acetylcholine receptors, and muscle activity reappears.
Purpose: to compare the efficacy and safety of sugammadex and neostigmine in reversing rocuronium-induced muscle relaxation to reach complete recovery of neuromuscular block (TOF ratio≥ 0.9) in infants undergoing inguinal hernia repair.
Methods: sixty infants aged from one month to one year of life, ASA I of both sexes and scheduled for elective inguinal hernia repair under general anesthesia were divided into two equal groups, 30 patients each. Group S received sugammadex in a dose of 2.0 mg/kg IV, while Group N received 0.02 mg/kg atropine with neostigmine 0.05 mg/kg IV to reverse the effects of rocuronium. Heart rate and mean arterial blood pressure were recorded at the baseline before induction of general anesthesia, intraoperatively every 15 minutes, and postoperatively every 30 minutes during the first two hours of stay in Post Anesthesia Care Unit (PACU). Reversal of neuromuscular blockade (NMB) was assessed by recording the time from administration of sugammadex or neostigmine till TOF ratio reaches 0.9 using TOF Watch®. Adverse events were recorded. Evaluation of recovery by recording the time for modified Aldrete score to reach 10 and modified pain-discomfort scale.
Results: time from administration of the study drugs till TOF ratio reaches 0.9 was significantly shorted in group S than in group N (mean=1.2 vs 9.2 min). There was significant decrease in group S compared to group N in regard of time from discontinuation of inhalational anesthesia till the patient reaches score 10 in modified Aldrete score in the PACU (minutes) (mean of 21.2± 7.7 vs 26.0± 9.3 minutes respectively). Postoperatively, the heart rate was statistically significantly higher in group N relative to group S during the first 30 minutes postoperatively.
Conclusions: Sugammadex administration for reversal of rocuronium-induced NMB in infants in a dose of 2 mg/kg results in a faster recovery of TOF ratio 0.9 than neostigmine (0.05 mg/kg) with atropine (0.02 mg/kg) with no major side effects.
Keywords: Sugammadex, neostigmine, rocuronium, inguinal hernia repair
INTRODUCTION:
Neuromuscular blocking agents (NMBAs) are frequently used during general anesthesia to facilitate tracheal intubation, artificial ventilation and surgical procedures. Rocuronium is a non-depolarizing aminosteroidal neuromuscular blocking agent with a rapid to intermediate onset of action and an intermediate duration of effect1.
Several cholinesterase inhibitors such as neostigmine, pyrido-stigmine, and edrophonium are commonly used to speed the reversal of neuromuscular blockade2. Among these agents, neostigmine is the most potent and selective one3. Neostigmine IV dose of 0.03–0.07 mg/kg usually is sufficient to achieve adequate recovery of neuromuscular function (i.e., TOF ratio 0.9) within 10–20 minutes.
It should be kept in mind that cholinesterase inhibitor agents have side effects such as bradycardia, QT lengthening, bronchoconstriction, hypersalivation and increased motility. Since these agents are not selective to nicotinic receptors and also stimulate the muscarinic system3. To avoid these effects, concomitant administration of anticholinergic agents, such as atropine or glycopyrrolate, are administered to the patient with the cholinesterase inhibitors3.
Sugammadex, a modified gamma-cyclo-dextrin, is a selective relaxant-binding agent which rapidly and completely reverses the effects of rocuronium by encapsulating and rendering it inactive4. Sugammadex does not bind to muscarinic receptors, it has the advantage that it is not associated with the side effects that may occur with the use of cholinesterase inhibitors5.
Pediatric patients differ from adult patients because the pharmaco-kinetic and pharmacodynamic profiles of NMBAs may vary as a function of age6. For example, the clinical duration of rocuronium is prolonged in infants compared with children7,8 and the potency of rocuronium is greater in infants and less in children as compared to adults9. Neostigmine takes a shorter time to reverse the blockade in infants than in children or adolescents10.
Despite being in the market since 200611,12, sugammadex is still not licensed for use in pediatric patients younger than 2 years despite recent review of clinical indications by Food and Drug Administration in the USA12, and there are no sufficient randomized controlled studies to support such licensing. All evidence present currently is case series and case reports13–16. Further research is needed to establish its safety and efficacy in this age group.
AIM OF WORK:
The study is comparing the efficacy of sugammadex versus neostigmine in reversing rocuronium-induced muscle relaxation to reach complete recovery of neuromuscular block (TOF ratio≥ 0.9) in infants undergoing inguinal hernia repair under general anesthesia in our center, comparing this to the already proven efficacy of sugammadex in adult patients.
METHODS:
After approval of the Ethical Committee of the Faculty of Medicine, Alexandria University, Egypt, a written informed consent was be obtained from each patient’s parents or guardian. The present study was carried out in the Alexandria Main University Hospitals. Sixty infants, aged from one month to one year of life, American Society of Anesthes-iologists (ASA) class I of both sexes and scheduled for elective inguinal hernia repair under general anesthesia, were included.
Patients were divided into two equal groups (thirty patients each) in a randomized manner (closed envelope method) according to the drug used to reverse the muscle relaxant:
Group N: patients will receive 0.02 mg/kg atropine with neostigmine 0.05 mg/kg IV.
Group S: patients will receive sugammadex 2mg/kg IV.
All the patients were assessed pre-operatively through detailed medical and surgical history, complete clinical examination and routine laboratory investigations. On arrival at the operating room, patients were monitored using standard ASA monitoring; electrocardiograph, a non-invasive arterial blood pressure monitor and pulse oximeter.
Patients aged <7 months were premedicated with oral midazolam. Anesthesia was induced in both groups with 4% sevoflurane with oxygen via face mask. After achieving a sufficient depth of anesthesia (assessed through loss of eye lash reflex), an intravenous access was obtained to administer 2 mcg/kg of fentanyl, 4 mg mg/kg of propofol and 0.6 mg/kg of rocuronium (after assessing TOF as outlined below).
The first TOF ratio was calibrated and measured using TOF Watch® (Organon Teknika, Eppelheim, Germany) that was placed on the ulnar nerve. The negative electrode was placed at the level of the wrist on the ulnar surface at the flexor crease. The positive electrode was placed 1-2 cm proximal to the negative, parallel to the flexor carpi ulnaris tendon. With this placement of the electrodes, electrical stimulation normally elicited finger flexion and thumb adduction, then patients received 0.6 mg/kg rocuronium. The dose was calculated based on lean body weight. The patients were orally intubated 90 seconds after the first dose of rocuronium.
Demographic data, such as age, gender, and body weight were recorded for all patients. Heart rate and mean arterial blood pressure were also recorded as a baseline before induction of general anesthesia, intraoperatively every 15 minutes, and postoperatively every 30 minutes during the first two hours in Post Anesthesia Care Unit (PACU). After completion of the surgery, the study drug was given based on patient allocation. Assessment of reversal was done by recording the time (in seconds) from time of administration of sugammadex or neostigmine till TOF ratio reaches 0.9 using the TOF Watch®. Only then was the sevoflurane switched off allowing patients to wake up. Adverse events, such as upper airway obstruction, hypoventilation, hypercapnia, hypoxia, acute respiratory failure (reflecting recurarization, among other causative factors), post-operative nausea and vomiting, and heart rate disturbances (bradycardia, tachycardia and arrhythmias), were recorded. Evaluation of quality of recovery was assessed by recording the time for modified Aldrete score to reach 10 and modified pain-discomfort scale.
RESULTS:
There were no statistically significant differences between both groups as regards age, sex and body weight (Table 1).
| Age (months) | Sex | Wt. (kg) | ||||
| S | N | S | N | S | N | |
| Min | 1 | 1 | 29 M 96.7% | 28 M 93.3% | 4.5 | 4 |
| Max | 12 | 12 | 12 | 11 | ||
| Mean | 6.47 | 6.8 | 1 F 3.3% | 2 F 6.7% | 7.55 | 7.43 |
| ±S.D. | 3.8 | 3.46 | 2.19 | 2.23 | ||
| Test of sig. | t= 0.355 | c2 = 0.351 | t = 0.204 | |||
| p | 0.724 | 0.554 | 0.839 | |||
Table 1: Demographic data of the groups studied. t, p: t and p values for Student t-test. χ2, p: χ2 and p values for Chi square test
Comparison between Group S and Group N showed no statistical difference regarding total dose of muscle relaxant administered (mean= 4.6 ± 1.5 vs 4.5 ± 1.3 mg rocuronium respectively) (Figure 1).
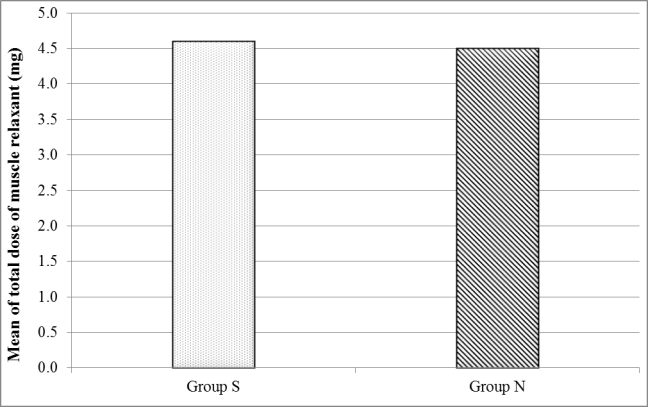
Figure 1: Comparison between the different studied groups according to total dose of muscle relaxant (mg).
Assessment of reversal (time from administration of the study drug till TOF ratio reaches 0.9) was faster with group S than with group N (mean=1.2 ± 0.3 vs 9.2 ± 0.9 min, p <0.001) with a significant difference between both groups (Figure 2).

Figure 2: Comparison between the different studied groups according to time to achieve of TOF ratio of 0.9 (minutes).
There was a significant decrease in group S compared to group N as regards the time from dis-continuation of inhalational anesthesia till the patient reaches score 10 in modified Aldrete score in the PACU (minutes). Modified pain discomfort scale showed no statistically significant difference between groups (Figures 3 and 4).

Figure 3: Comparison between the studied groups regarding time for modified Aldrete score to reach 10 (minutes).
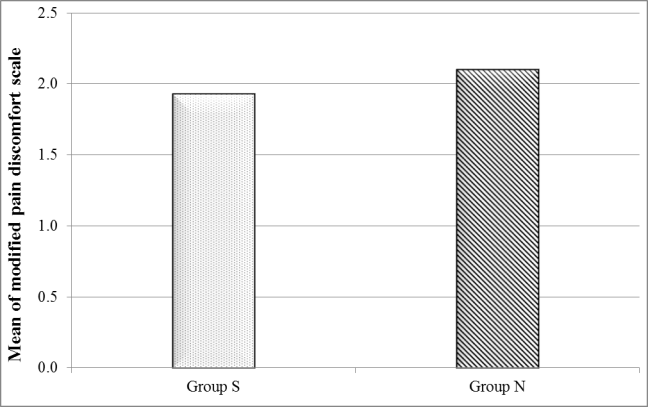
Figure 4: Comparison between the studied groups regarding modified pain-discomfort scale.
Regarding the incidence of adverse events, there were two patients who developed post-operative nausea and vomiting in group S and four patients developed post-operative nausea and vomiting in group N. Yet, there was no significant difference between the groups (Figure 5).
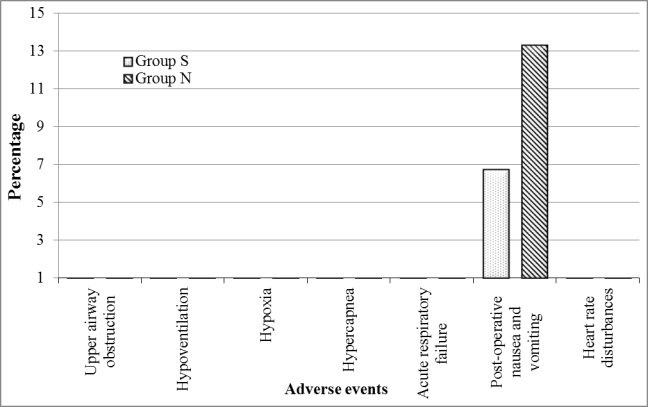
Figure 5: Comparison between the different studied groups according to incidence of adverse events.
DISCUSSION:
Despite being available on the market since 2006, sugammadex remains unlicensed for use in pediatric patients younger than 2 years old. This restriction persists even after a recent review of clinical indications by the Food and Drug Administration (FDA) in the USA in 202112. The efficacy and superiority of sugammadex to other reversal agents have been proved through numerous studies, however, little is known about that in young children and infants17,18.
This is largely because of lack of sufficient randomized controlled trials (RCTs) to support its use in this age group. RCTs are considered the gold standard in clinical research as they provide the most reliable evidence on the efficacy and safety of medical interventions. However, conducting RCTs in pediatric populations, particularly those under 2 years of age, presents significant ethical and logistical challenges. These include the difficulty in obtaining informed consent, the variability in drug metabolism and effects in young children, and the small number of patients available for study.
Our study was designed to compare the efficacy of sugammadex and neostigmine in the reversing of rocuronium-induced muscle relaxation to reach complete recovery of neuromuscular block (TOF ratio≥ 0.9) in infants undergoing inguinal hernia repair, supporting its use in such age group. Efficacy was measured by assessing the time needed to achieve TOF 0.9 and more, along with a good safety profile and lack of major side effects (listed in methods).
In our study, the time from the start of administration of sugammadex or neostigmine (reappearance of T2) to recovery of the TOF ratio to 0.9 was significantly shorter with the sugammadex group (S) than with the neostigmine group (N). The recurrence of block (also known as recurarization) was not observed in either group according to the clinical observation and follow up on oxygen saturation in the PACU. All studies conducted on pediatric patients with different doses of sugammadex (2, 3, and 4 mg/kg), in comparison with neostigmine for the reversal of rocuronium induced NMB, agree with our findings, and report that the administration of sugammadex made faster NMB reversal possible, when compared with traditional drugs as neostigmine.
El Sayed and Hassan19 divided randomly 70 patients planned for outpatient total bilateral tonsillectomy into 2 groups. Group S (n=35) received 2 mg/kg sugammadex to reverse NMB achieved by rocuronium. Group N (n= 35) received 0.05 mg/kg neostigmine and atropine sulfate 0.01 mg/kg. They found TOF ratio after reversal was statistically less in group S than in group N. The time when TOF ratio exceeded 0.9 and extubation time were significantly less in group S than in group N. No adverse effects were recorded in both groups.
Plaud et al.20 compared the efficiency and safety of sugammadex in infants, children, adolescents, and adults. A dose-response relation was demonstrated in children (n = 22), adolescents (n = 28), and adults (n = 26), but not infants (n = 8). They attributed this to the small sample size of the infant group. They reported that after placebo, the median recovery time of TOF to 0.9 was 21 and 19 min in infants and children, respectively. After 2.0 mg/kg sugammadex TOF of 0.9 was attained in 0.6 and 1.2 min in infants and children, respectively.
A randomized prospective study conducted by Kara et al.21 who studied 80 patients aged 2-12 years, scheduled for elective lower abdominal surgical procedures. Neuromuscular blockade was achieved with 0.6 mg/kg rocuronium and monitored with TOF. Group N (n = 40) received 0.03 mg/kg neostigmine, Group S (n = 40) received 2 mg/kg sugammadex for reversal of NMB. Extubation time and time to reach TOF >0.9 were significantly higher in Group N than in the S group. No side effects occurred in either of the groups.
Zaini et al.22 found that there was a significantly faster recovery time from TOF count 2 or 3 to TOF ratio 0.9 post-reversal in the sugammadex group (2 mg/kg), with a mean of 1.4 ± 0.7 minutes as compared to only 8.36 ± 1.9 minutes in the neostigmine group (0.05 mg/kg of neostigmine with 0.02 mg/kg of atropine). The time from reversal to extubation was also significantly shorter in the sugammadex group with mean time of 1.76 ± 0.55 minutes compared to neostigmine 11.88 ± 2.2 minutes.
Guzelce et al.23 compared time to recovery to the TOF ratio of 0.9 and the extubation time between sugammadex and neostigmine in thirty-seven pediatric patients undergoing lower urinary tract surgery and inguinal hernia. The patients were randomized into two groups: group N received neostigmine 0.05 mg/kg and group S received sugammadex 2 mg/kg. Extubation time was found to be statistically longer in group N than in group S.
Veiga et al.24 included 24 patients aged 2-9 years, scheduled for elective surgery under general anesthesia. They randomized the patients to receive either Sugammadex 2mg/kg (n=14) or neostigmine 0.05 mg/kg and atropine 0.025 mg/kg (n=10). The mean time to reach a 0.9 TOF ratio was significantly shorter in sugammadex 1.06 ± 0.3, and 11.5 ± 7.4 min than in neostigmine group.
Ozmete et al.25 conducted a study on twenty-six infants (2-12 months of age) who were scheduled to undergo neurosurgical procedures. Patients received 3 mg/kg sugammadex. The mean recovery time of the T4/T1 ratio of 0.9 was 112.65 ± 35.60 seconds.
Benigni et al.26 conducted a study to assess the efficacy of a sugammadex dose of 4mg/kg in early reversal of a rocuronium-induced NMB in pediatric patients. Thirty-four children, aged from 2 months to 8 years scheduled for brief medical procedures requiring general anesthesia were enrolled. At the end of the procedure all children enrolled achieved TOF of 0.9 after receiving sugammadex 4mg/kg. The recovery time was 104 ± 47 seconds.
A study done by Ghoneim et al.27 on 40 pediatric patients undergoing elective craniotomy for posterior fossa tumor excision were randomly divided into either neostigmine (0.04 mg/kg added to atropine 0.02 mg/kg) or sugammadex (4 mg/kg) group. The patients in the sugammadex group attained a TOF ratio of 0.9 in a statistically significant shorter time (1.4 ± 1.2 min) than those in neostigmine group (25.16 ± 6.49 min) for reversal of the rocuronium.
Alonso et al.28 conducted a study on 23 neonates. The patients were divided into two age groups, 1-day (n=8), mean weight 2.8 ± 0.1 kg and 1-7 days (n=15), mean weight 2.4 ± 0.8 kg respectively. Sugammadex was administered in a dose of 4.0 mg/kg. The time to reach TOF ratio to 0.9 was 1.4 ±0.1 min in the 1-day group and 1.2 ±0.5 min in the 1-7 days group.
Ammar and his colleagues29 who enrolled sixty pediatric patients aged 2-10 years scheduled for lower abdominal surgeries were randomly assigned into two equal groups to receive 4 mg/kg sugammadex (Group S) or 0.35 mg/kg neostigmine and 0.02 mg/kg atropine (Group N) as a reversal agent for rocuronium at the end of surgery, found that no patient in either the sugammadex or neostigmine groups developed residual NMB. Also, Ozmete et al.25 reported that there was no clinical evidence of residual curarization observed in infants who received 3 mg/kg sugammadex.
In the present study, the mean time from discontinuation of inhalational anesthesia till the patient reached a score of 10 in the modified Aldrete score in the PACU was 21.2 ± 7.7 minutes among group S, and 26.0 ± 9.3 minutes among group N showing significant shorter duration in group S. Özgün et al.30 found no significant difference regarding respiratory parameters between their studies’ two groups (group 1 receiving 2 mg/kg sugammadex for reversal of rocuronium NMB and group 2 receiving 0.06 mg/kg neostigmine +0.02 mg/kg atropine), while the state of consciousness revealed a significant difference only at 10th and 15th min after reverse between groups23. Ammar and colleagues29 reported that the time from reversing agent injection to PACU arrival was significantly shorter in group S than group N.
In the present study, the highest percentage of both group S and group N got score 2 in the modified Aldrete score in the PACU (40%, and 46.7% respectively) with no statistically significant difference between group S and group N. Clinical studies have included only a limited number of infants under 2 years of age. Consequently, there is insufficient data to determine the efficacy or optimal dosing of sugammadex in this population. Given the scarcity of studies focused specifically on infants, we compared our findings with those from studies involving both infants and children.
In the present study, the incidence of post-operative nausea and vomiting was not significantly different between study groups (n=2, 6.7% in group S, n=4, 13.3% in group N). This result is compatible with many studies20,22,31.
Abrishami et al.31 did a systematic review in 2009 which included all RCTs in which sugammadex was compared with placebo or other medications, or in which different doses of sugammadex were compared with each other to assess the efficacy and safety of sugammadex in reversing neuromuscular blockade induced by steroidal NDMRs and reported no significant difference between sugammadex and neostigmine receiving groups regarding the adverse effects.
Plaud et al.20 reported that only one child experienced vomiting by 2 mg/kg sugammadex, while Ghoneim et al.27 reported that two patients (10%) in the sugammadex group and 4 (20%) in the neostigmine group developed postoperative vomiting.
The incidence of complications post-reversal reported by Zaini et al22, was higher in the neostigmine group with 17.5% (7 patients) developed postoperative nausea and vomiting, no complications were noted in the sugammadex group15.
Ammar et al.29 reported that incidence of post-operative nausea, vomiting and tachycardia were significantly lower in Group S and no patient in either group developed bradycardia or respiratory depression.
CONCLUSION:
From our study we concluded that sugammadex administration in a dose of 2 mg/kg results in a faster reversal of rocuronium NMB, and faster achieving of TOF ratio 0.9 than neostigmine (0.05 mg/kg) with atropine (0.02 mg/kg). Sugammadex administration for reversal of rocuronium-induced NMB in infants resulted in shorter extubation times with no residual curarization. Sugammadex administration has lower incidence of PONV, and tachycardia when compared with neostigmine and atropine.
This study adds to the growing body of evidence that supports licensing sugammadex for pediatric use in patients younger than 2 years. While almost all the studies examined during our literature review were conducted on small cohorts, and the younger the intended group of patients the number gets smaller, it is worth noting that no major safety concerns were raised by any of them. We recommend that more studies to be conducted studying the efficacy and safety of sugammadex on children under 2 years of age, especially infants and neonates, exploring different surgeries and clinical scenarios, such as emergency intubation in critical care settings.
Acknowledgement
The authors declare no conflict of interest, and no external funding. Informed consent was taken from all patients prior to publishing.
References:
- Baillard C, Clec’h C, Catineau J, et al. Postoperative residual neuromuscular block: a survey of management. Br J Anaesth. 2005;95(5):622-626. doi:10.1093/BJA/AEI240
- Bowman WC. Neuromuscular block. Br J Pharmacol. 2006;147 Suppl 1(Suppl 1). doi:10.1038/SJ.BJP.0706404
- Naguib M, Lien CA. Pharmacology of Muscle Relaxants and Their Antagonists. In: Miller R, ed. Miller’s Anesthesia. 6th ed. Churchill Livingstone; 2006:481-572.
- Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27(10):874-881. doi:10.1097/EJA.0B013E32833D56B7
- Suy K, Morias K, Cammu G, et al. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106(2):283-288. doi:10.1097/00000542-200702000-00016
- Peeters PAM, van den Heuvel MW, Heumen E van, et al. Safety, tolerability and pharmacokinetics of sugammadex using single high doses (up to 96 mg/kg) in healthy adult subjects: a randomized, double-blind, crossover, placebo-controlled, single-centre study. Clin Drug Investig. 2010;30(12):867-874. doi:10.1007/BF03256915
- De Boer HD, Driessen JJ, Marcus MAE, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose-finding and safety study. Anesthesiology. 2007;107(2):239-244. doi:10.1097/01.ANES.0000270722.95764.37
- Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104(3):555-562. doi:10.1213/01.ANE.0000260135.46070.C3
- Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109(5):816-824. doi:10.1097/ALN.0B013E31818A3FEE
- Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111(1):110-119. doi:10.1213/ANE.0B013E3181C07428
- Committee for Medicinal Products for Human Use (CHMP). Bridion, INN-Sugammadex.; 2015. Accessed August 5, 2024. http://www.ema.europa.eu/contact
- FDA. HIGHLIGHTS OF PRESCRIBING INFORMATION – BRIDION. Published online 2021. Accessed August 5, 2024. http://www.fda.gov/medwatch.
- Franz AM, Chiem J, Martin LD, Rampersad S, Phillips J, Grigg EB. Case series of 331 cases of sugammadex compared to neostigmine in patients under 2 years of age. Paediatr Anaesth. 2019;29(6):591-596. doi:10.1111/pan.13643
- Efune PN, Alex G, Mehta SD. Emergency Sugammadex Reversal in an 850-G Premature Infant: A Case Report. The Journal of Pediatric Pharmacology and Therapeutics : JPPT. 2021;26(1):107. doi:10.5863/1551-6776-26.1.107
- Kim H, Cho J, Lee S, Lim Y, Yoo B. The use of sugammadex in an infant with prolonged neuromuscular blockade – A case report. Anesth Pain Med (Seoul). 2022;17(1):52-56. doi:10.17085/APM.21071
- Kim H, Cho J, Lee S, Lim Y, Yoo B. The use of sugammadex in an infant with prolonged neuromuscular blockade – A case report -. Anesth Pain Med (Seoul). 2022;17(1):52. doi:10.17085/APM.21071
- Liu G, Wang R, Yan Y, Fan L, Xue J, Wang T. The efficacy and safety of sugammadex for reversing postoperative residual neuromuscular blockade in pediatric patients: A systematic review. Sci Rep. 2017;7(1). doi:10.1038/S41598-017-06159-2
- Lang B, Han L, Zeng L, et al. Efficacy and safety of sugammadex for neuromuscular blockade reversal in pediatric patients: an updated meta-analysis of randomized controlled trials with trial sequential analysis. BMC Pediatr. 2022;22(1):1-12. doi:10.1186/S12887-022-03288-0/TABLES/2
- El sayed M, Hassan S. Does sugammadex facilitate recovery after outpatient tonsillectomy in children? Egypt J Anaesth. 2016;32(4):447-450. doi:10.1016/J.EGJA.2016.08.021
- Plaud B, Meretoja O, Hofmockel R, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110(2):284-294. doi:10.1097/ALN.0B013E318194CAAA
- Kara T, Ozbagriacik O, Turk HS, et al. [Sugammadex versus neostigmine in pediatric patients: a prospective randomized study]. Rev Bras Anestesiol. 2014;64(6):400-405. doi:10.1016/J.BJAN.2014.03.001
- Mohamad Zaini RH, Penny Tevaraj JM, Wan Hassan WN, Iberahim MI, Wan Muhd Shukeri WF. Abstract PR253: Comparison Between the Efficacy of Neostigmine Versus Sugammadex for Reversal of Rocuronium Induced Neuromuscular Blockade in Paediatric Patients. Anesth Analg. 2016;123:329. doi:10.1213/01.ANE.0000492650.20862.E4
- Güzelce D, Kendigelen P, Tütüncü AÇ, Kaya G, Altıntaş F. [Comparison of Sugammadex and Neostigmine in Terms of Time to Extubation in Pediatrics]. Haseki Tip Bulteni. 2016;54(4):207-211. doi:10.4274/HASEKI.3091
- Veiga RG, Carceles BMD, Dominguez SN, Lopez FL, Orozco MJ, Alvarez-Gomez JA. Sugammadex reversal efficacy and security vs neostigmine in the rocuronium – induced neuromuscular blockade in paediatric patients 10AP3-5. Eur J Anaesthesiol. 2011;28:153-153.
- Ozmete O, Bali C, Cok OY, et al. Sugammadex given for rocuronium-induced neuromuscular blockade in infants: a retrospectıve study. J Clin Anesth. 2016;35:497-501. doi:10.1016/J.JCLINANE.2016.08.040
- Benigni A, Maffioletti M, Spotti A, Benigni AM, Locatelli BG, Sonzogni V. Efficacy and safety of a sugammadex dose of 4 mg/kg in early reversal of a deep neuromuscular block rocuronium-induced in infants and children: a case series. Eur J Anaesthesiol. 2013;30:161-162.
- Ghoneim AA, El Beltagy MA. Comparative study between sugammadex and neostigmine in neurosurgical anesthesia in pediatric patients. Saudi J Anaesth. 2015;9(3):247-252. doi:10.4103/1658-354X.154696
- Alonso A, de Boer HD, Booij L. Reversal of rocuronium-induced neuromuscular block by sugammadex in neonates 10AP1-3. Eur J Anaesthesiol. 2014;31:163-163.
- Ammar AS, Mahmoud KM, Kasemy ZA. A comparison of sugammadex and neostigmine for reversal of rocuronium-induced neuromuscular blockade in children. Acta Anaesthesiol Scand. 2017;61(4):374-380. doi:10.1111/AAS.12868
- Özgün Ç, Çakan T, Baltacı B, Başar H. Comparison of reversal and adverse effects of sugammadex and combination of — Anticholinergic-Anticholinesterase agents in pediatric patients. J Res Med Sci. 2014;19(8):762. Accessed August 6, 2024. /pmc/articles/PMC4235098/
- Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009;(4). doi:10.1002/14651858.CD007362.PUB2
For citation:
Tawfiq Z., Yacout A., El-Hadi S., (2024), Sugammadex Versus Neostigmine for Reversal of Rocuronium-Induced Muscle Relaxation in Infants Undergoing Inguinal Hernia Repair: a randomized controlled study. MEJMMS, 1(1), 1013-24; mejmms.org /2024/10/17/sugammadex-versus-neostigmine-for-reversal-of-rocuronium-induced-muscle-relaxation-in-infants-undergoing-inguinal-hernia-repair-a-randomized-controlled-study/

Leave a comment